
A heat exchanger is a device designed to facilitate the transfer of thermal energy (heat) from one fluid to another. Heat exchangers are ubiquitous in industry, being used in a very wide range of applications from food sterilisation to power generation. As a result, heat exchanger technology has evolved over the years, driven by the need for ever more efficient processes.
An important type of heat exchangers is the plate heat exchanger. This heat exchanger is made from metal plates that are joined together to form the heat exchanger unit. The process fluid and the heating/cooling fluid are allowed to flow within separate channels, allowing heat to be transferred from the hot fluid to the metal plates, and then from the metal plates to the colder fluid. The flow channels can be created using photochemical etching – a process that removes metal selectively from a metal plate using a chemical reagent. This report aims to demonstrate how the photochemical etching technique can be employed in the manufacture of plate heat exchangers, and how it can be useful in the optimisation of the design of such heat exchangers. But first, let us take a brief look at how heat is transferred between two fluids.
Role Of Metals In Thermal Conduction
On a molecular level, heat is a measure of the average kinetic energy of the molecules that make up a material (that is, the energy due to the movement of the molecules). In fluids (i.e. liquids and gases), molecules are in constant motion, and the faster they move, the higher their kinetic energy and the higher the temperature. The molecules of a solid, in contrast, do not move from one point to another; instead, they vibrate. The higher the temperature of the solid, the faster its molecules or atoms vibrate and the bigger the amplitude of the vibrations.
To transfer heat from a hot fluid to a colder fluid, we need a method of transferring the kinetic energy of the fast-moving molecules of the hot fluid to the slower-moving molecules of the colder fluid. This can be achieved through the use of a medium that can absorb thermal energy from the hot fluid and deliver it to the colder fluid. The medium must be (i) in the solid state so that it forms a physical barrier between the two fluids, (ii) chemically passive towards the two fluids so that it does not react with them, and (iii) a good conductor of heat. A certain class of materials fits the bill perfectly, namely metals. Metals are solid and remain in the solid state at high temperatures; several metals have excellent chemical resistance thanks to their protective oxide layer; and finally, metals are excellent conductors of heat.
The high thermal conductivity of metals is a result of a special feature of their atomic structure. In the solid state, the atoms of a metal join together in a way that allows each atom to donate one, two, or three of its electrons to form a sea of electrons that are free to move within the metallic structure, while the atom itself turns into a positive ion. This sea of delocalized electrons is central to many of the useful properties of metals. It allows metals to conduct electricity and heat and it gives metals a strong metallic bond. When one end of a metallic bar is heated, the heat causes the free electrons in the heated part of the bar to move faster. These high-energy electrons then collide with slower electrons and also with the positive ions of the metal. With each collision, a fast-moving electron transfers part of its energy to a slower-moving electron or to a metal ion. As a result, the electrons move faster and the ions vibrate more. The vibrations spread throughout the metal, heating up the entire metal bar.
The same mechanism operates when one side of a metal sheet is in contact with a fluid at high temperature while the other side is in contact with a fluid at a lower temperature. Heat is transferred from the hot fluid to the metal, and then from the metal to the colder fluid. The larger the contact area between the fluids and the metal, the higher the rate of heat transfer. Heat exchangers utilise this mechanism to allow hot fluids to transfer their heat to colder fluids through a metal medium.
The rate at which heat is conducted through a metal varies from metal to metal. Silver, copper and gold, for example, have very high thermal conductivities at 420, 390 and 318 W/m°C, respectively. Aluminium – an attractive metal in many applications due to its light weight – is also an excellent thermal conductor at 220 W/m°C, while stainless steel has one of the lowest thermal conductivities among metals at 14 W/m°C, but this is still significantly higher than the conductivity of most non-metals. For comparison, the thermal conductivity of water is 0.6 W/m°C.
Plate Heat Exchangers
On a molecular level, heat is a measure of the average kinetic energy of the molecules that make up a material (that is, the energy due to the movement of the molecules). In fluids (i.e. liquids and gases), molecules are in constant motion, and the faster they move, the higher their kinetic energy and the higher the temperature. The molecules of a solid, in contrast, do not move from one point to another; instead, they vibrate. The higher the temperature of the solid, the faster its molecules or atoms vibrate and the bigger the amplitude of the vibrations.
To transfer heat from a hot fluid to a colder fluid, we need a method of transferring the kinetic energy of the fast-moving molecules of the hot fluid to the slower-moving molecules of the colder fluid. This can be achieved through the use of a medium that can absorb thermal energy from the hot fluid and deliver it to the colder fluid. The medium must be (i) in the solid state so that it forms a physical barrier between the two fluids, (ii) chemically passive towards the two fluids so that it does not react with them, and (iii) a good conductor of heat. A certain class of materials fits the bill perfectly, namely metals. Metals are solid and remain in the solid state at high temperatures; several metals have excellent chemical resistance thanks to their protective oxide layer; and finally, metals are excellent conductors of heat.
The high thermal conductivity of metals is a result of a special feature of their atomic structure. In the solid state, the atoms of a metal join together in a way that allows each atom to donate one, two, or three of its electrons to form a sea of electrons that are free to move within the metallic structure, while the atom itself turns into a positive ion. This sea of delocalized electrons is central to many of the useful properties of metals. It allows metals to conduct electricity and heat and it gives metals a strong metallic bond. When one end of a metallic bar is heated, the heat causes the free electrons in the heated part of the bar to move faster. These high-energy electrons then collide with slower electrons and also with the positive ions of the metal. With each collision, a fast-moving electron transfers part of its energy to a slower-moving electron or to a metal ion. As a result, the electrons move faster and the ions vibrate more. The vibrations spread throughout the metal, heating up the entire metal bar.
The same mechanism operates when one side of a metal sheet is in contact with a fluid at high temperature while the other side is in contact with a fluid at a lower temperature. Heat is transferred from the hot fluid to the metal, and then from the metal to the colder fluid. The larger the contact area between the fluids and the metal, the higher the rate of heat transfer. Heat exchangers utilize this mechanism to allow hot fluids to transfer their heat to colder fluids through a metal medium.
The rate at which heat is conducted through a metal varies from metal to metal. Silver, copper and gold, for example, have very high thermal conductivities at 420, 390 and 318 W/m°C, respectively. Aluminium – an attractive metal in many applications due to its light weight – is also an excellent thermal conductor at 220 W/m°C, while stainless steel has one of the lowest thermal conductivities among metals at 14 W/m°C, but this is still significantly higher than the conductivity of most non-metals. For comparison, the thermal conductivity of water is 0.6 W/m°C.

Printed circuit heat exchangers are a type of plate heat exchangers that can handle high pressures up to 600 bars or even higher, and temperatures from deep cryogenic to more than 800°C. They were developed at the University of Sydney in the early 1980s and commercialized in 1985 by Heatric in Australia. Two important techniques are used in producing these heat exchangers, photochemical etching and diffusion bonding.
Photochemical etching is used to create flow channels that are typically between 0.2mm and 3mm in width on flat metal plates. The narrow channels allow a large heat exchange surface area within a relatively small volume. The etching technique is similar to the process used to manufacture electronic printed circuit boards, hence the name given to this type of heat exchangers. The flow channels produced by etching have a semi-circular cross section. The plates can be manufactured in a range of high-performance alloys such as austenitic stainless steels, nickel-based alloys, copper and titanium.
The etched plates are stacked and then joined together using diffusion bonding. This is a solid-state joining process that works by applying heat (at temperatures in excess of 60% of the melting point of the metal) and pressure in a controlled atmosphere to allow grain growth across the two metal surfaces being joined. The resulting joint exhibits the strength and ductility of the parent metal.
Printed circuit heat exchangers are used in applications such as natural gas processes, gas turbines, and hydrogen fueling stations. Two major factors affecting the thermal performance of these heat exchangers are the geometry (e.g. wavy or zigzag shaped) and dimensions of the flow channels, and the type of flow employed (parallel, counter or cross flow). During the development stage of the heat exchanger for a particular application, the speed and low-cost tooling of the photochemical etching process allows different channel geometries and flow types to be tested. Experimentation with different designs can be achieved at a low cost.
The flow channels produced by photochemical etching usually have a semi-circular cross section, as shown in Figure 1. The figure shows a cross section through an etched plate with multiple flow channels. A more detailed case study is presented in the next section.
Figure 2: Flow channels on a stainless steel heat exchanger plate (20× magnification)
Advanced Chemical Etching Ltd. (ACE) manufactures heat exchanger plates in different sizes, metals and geometries. In particular, ACE can produce high quality heat exchanger plates in four classes of materials: stainless steel, aluminium, copper and titanium alloys. Flow channels are created by selectively dissolving the metal in a controlled way during the photochemical etching process. First, a photosensitive polymeric film, known as the photoresist, is applied to the metal surface. The required plate design is then printed on a transparent polymeric film called the phototool. The phototool is used to transfer the image onto the photoresist-coated metal sheet using UV light. The photoresist is then selectively removed from the areas that require etching, thus exposing the bare metal in these areas. In the etching step, the bare metal is dissolved using a chemical reagent until the required dimensions are achieved. Finally, the photoresist layer is removed using a special chemical reagent. Figure 2 shows a section of a stainless steel plate with semi-circular channels. A more detailed view of these channels is shown in Figures 3 and 4.
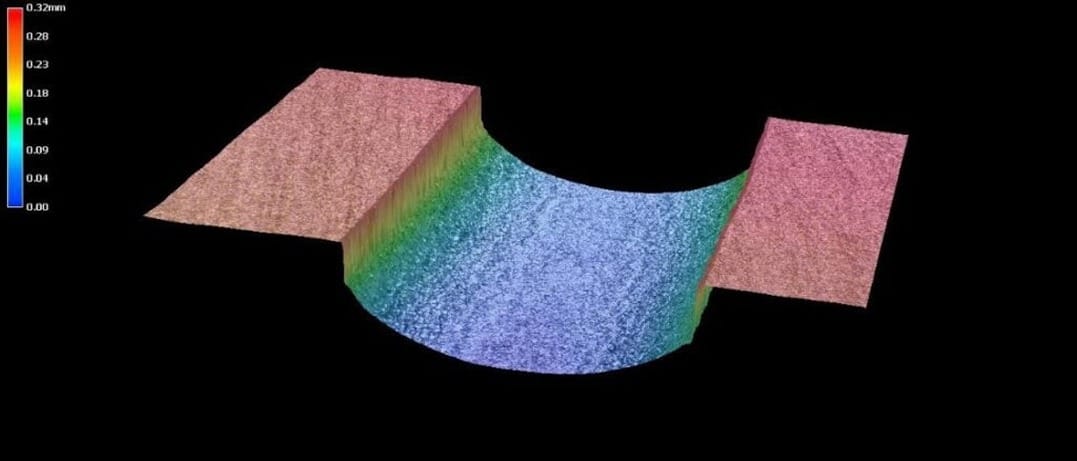
Figure 3: A cross-section of an etched flow channel at 200× magnification
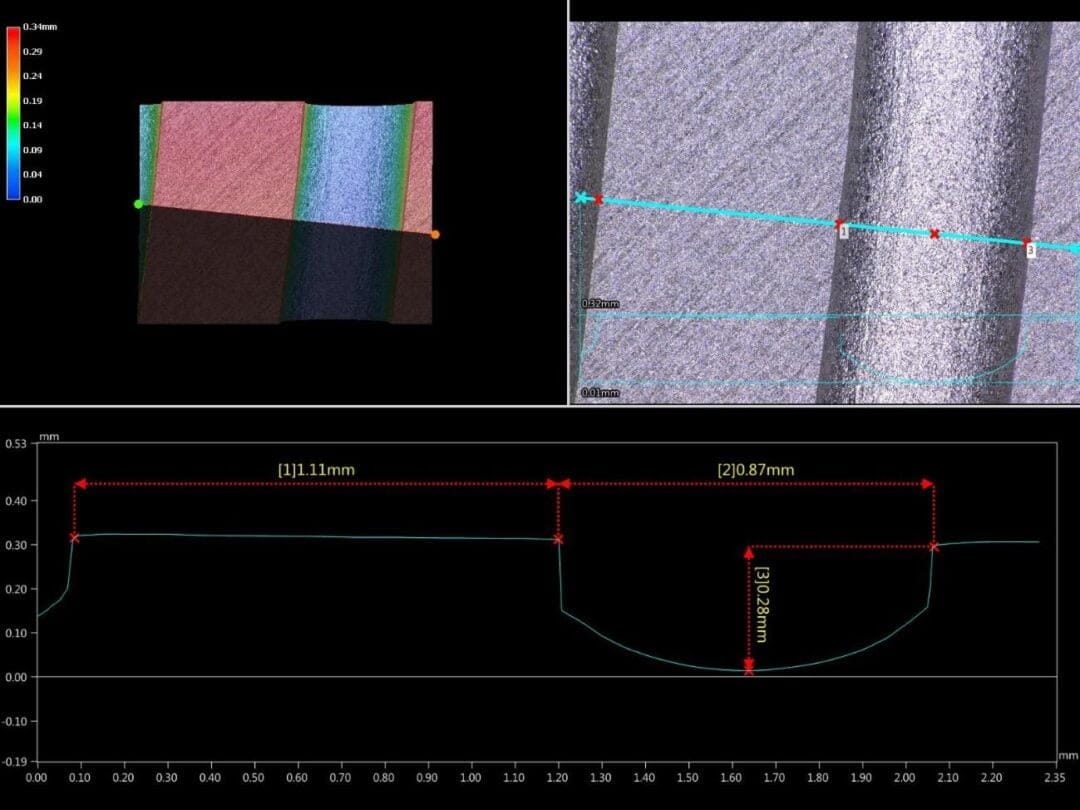
Figure 4: Measurements of channel and ridge dimensions (taken with a digital 3D microscope)
The channel and ridge dimensions are given in Figure 4. This figure also shows the typical cross section of the channels that can be produced by photochemical etching. In this case, the channel depth is 0.28mm, the channel width is 0.87mm and the ridge width between two adjacent channels is 1.11mm.
The channel and ridge dimensions have a big influence on the overall performance of the heat exchanger. The dimensions of the channels affect the heat transfer characteristics between the two fluids. The surface between adjacent channels – the ridge surface – can affect the bonding characteristics, as well as the flow and heat transfer characteristics. A smaller ridge width allows for a larger overall flow and heat transfer area on the plate, but reduces the available metal-to-metal contact area between the bonded plates. Optimisation of this dimension should yield the minimum acceptable ridge width that allows a sufficient bonding surface area without compromising the flow and heat transfer characteristics of the heat exchanger. Trials conducted at ACE show that a ridge width as narrow as 250µm can be produced consistently by photochemical etching.
The speed and low cost of the etching process mean that several design iterations can be produced and tested within a short period of time in order to arrive at the optimal plate design. Advanced measuring equipment such as CMM (co-ordinate measuring machine) and 3D microscopy can be used to measure the etched features accurately and within minutes (e.g. see Figures 3 & 4 above).
Moreover, advanced etching techniques developed at ACE now allow heat exchanger plates to be manufactured to a high level of quality in titanium and aluminium, two metals that are difficult to etch using conventional etching methods. The new etching processes use advanced analytical techniques such as x-ray fluorescence to monitor and control the etching chemistry, resulting in consistent etching and improved quality conformance.
At ACE, we combine cutting-edge chemical etching technology with decades of expertise to deliver precision metal components.
Whether you're an experienced engineer, new to metal etching, or just curious about what we do, we’ve got you covered.


